作者:罂粟花
可乐定与咪达唑仑作为术前用药对儿童术后不良行为改变的比较:一项随机对照试验
贵州医科大学 麻醉与心脏电生理课题组
翻译:宋雨婷 编辑:马艳燕 审校:曹莹
背景:术后不良行为改变(NBCs)在儿童中较为常见,但目前认为术前用药可降低该风险。多年来,咪达唑仑一直作为儿童的标准术前用药,而最近,α2肾上腺素能激动剂可乐定也作为麻醉前镇静剂用于临床麻醉。本试验使用术后行为量表(PHBQ)进行评估,并假设可乐定在限制儿童NBCs方面的作用优于咪达唑仑。
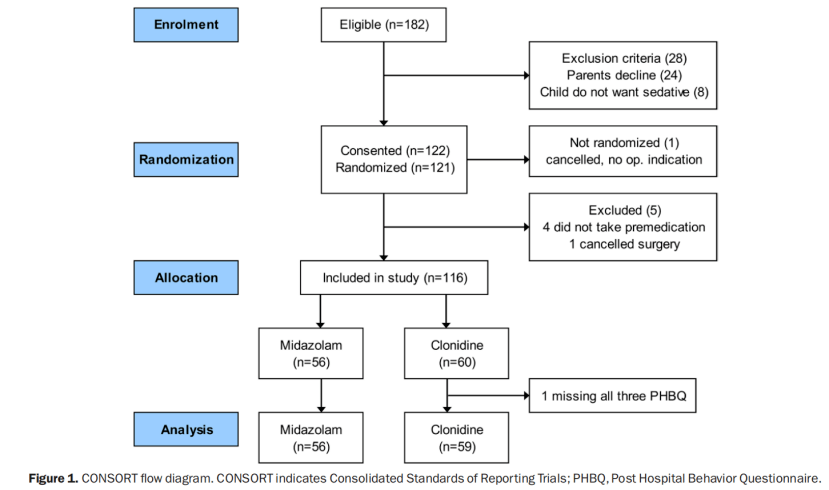
方法:本试验为一项前瞻性,随机,对照,盲法研究,纳入了115名儿童(年龄在24至95个月)及其父母。受试者接受了耳鼻喉门诊手术,并被随机分配到咪达唑仑组(术前口服0.5mg/kg)和可乐定组(术前口服4μg/kg)。参与者通过计划的方案进行麻醉。在患儿父母协助下完成术后1周,术后1个月和术后6个月PHBQ,用于术后NBCs的评估。次要指标之一为诱导前焦虑(使用改良耶鲁术前焦虑量表(mYPAS)进行评估)。
结果:1例患儿在术后1周发生超过3次NBCs,与咪达唑仑组相比,可乐定组的治疗比例没有差异(分别为12/59,20%比7/56,13%,比值比为1.39,95%置信区间[CI],0.75-2.58;P =0 .32)。与咪达唑仑组相比,可乐定组的患儿具有更高的诱导前焦虑水平(mYPAS>30,分别为43/59,71%比12/56,21%;P<0.001)。
结论:这些结果显示咪达唑仑和可乐定作为术前用药,在术后1周行为改变无显著临床或统计学差异。
原始文献来源:Zickerman, Caroline; Hult, Ann-Catrin; Hedlund, Lars,et al. Clonidine Versus Midazolam Premedication and Postoperative Negative Behavioral Changes in Younger Children: A Randomized Controlled Trial. Anesthesia & Analgesia: August 2022 - Volume 135 - Issue 2 - p 307-315
英文原文:
Clonidine Versus Midazolam Premedication and Postoperative Negative Behavioral Changes in Younger Children: A Randomized Controlled Trial
Background Postoperative negative behavioral changes (NBCs) are common among children, but risk for this is thought to be reduced with premedication. Midazolam has for many years been a standard premedication for children. More recently, the alpha-2 adrenergic agonist clonidine has also become popular as a preanesthetic sedative. We hypothesized that clonidine was superior to midazolam for limiting new NBCs in children as assessed using the Post Hospital Behavior Questionnaire (PHBQ).
Methods This was a prospective, randomized, controlled, blinded study, including 115 participants aged 24 to 95 months and their parents. The participants underwent ear, nose, or throat outpatient surgery and were randomly allocated to premedication with oral midazolam 0.5 mg/kg or oral clonidine 4 ?g/kg. Participants were anesthetized by protocol. At home, later, parents were asked to complete the PHBQ assessment instrument for postoperative NBCs for the participants 1 week, 1 month, and 6 months after the surgery. A secondary outcome, preinduction anxiety, was assessed using modified Yale Preoperative Anxiety Scale (mYPAS).
